Liquid CO2 and Liquid N2 comparison
Compare by1 KG (one kg)
Unit Conversion
1 psi = 0.0689 bar
1 kcal = 4.1868 kj
1. Liquid Nitrogen and Carbon Dioxide Properties
1.1 Liquid Nitrogen Thermal Properties
Boiling point -195.8°C
Latent heat 47.46kcal/kg (198.38kj/kg)
Specific heat 0.24kcal/kg (1.02kj/kg)
1.2 Liquid Carbon Dioxide Thermal Properties
Boiling point -78.9°C
Latent heat 136kcal/kg (570kj/kg)
Specific heat 0.21kcal/kg (0.88kj/kg)
Nitrogen
It is the major component of the air we breathe. Nitrogen is obtained as a liquid at a temperature of -195.8°C. To keep it in liquid form, it is stored in vacuum insulated tanks at low pressures (between 2 and 7 bar).
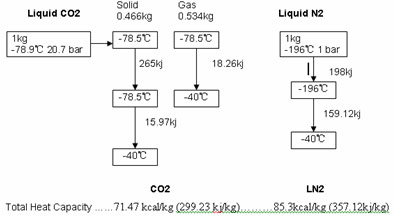
When liquid nitrogen is released to the atmosphere, it vaporizes and becomes gas. During this change of state it absorbs 47.46 kcal/kg (198kj/kg), which is the latent heat of fusion. The gas released at -196°C will warm up to a temperature at which it can no longer be used (-40°C for Remy use). The heat absorbed by this gas is called "sensible heat" and in this case its value is 38.00 kcal/kg (159.12kj/kg). For these values we obtained a LIN enthalpy of 85.3kcal/kg (357.12kj/kg).
Carbon Dioxide
Carbon dioxide in its liquid form is stored in pressurized vessels (between 15 and 20 bar). When released to atmospheric pressure it expands and become 46.66% solid CO2 and 53.34% cold gas, its boiling point being -78.9°C. The solid CO2 will sublimate absorbing 63.56 kcal/kg (265 kj/kg) in this isothermal process. The remaining gas at -78.9°C will warm up to the final temperature (-40°C for Remy use) and will absorb 12.6 kcal/kg (52.66kj/kg), which is the sensible heat. The total carbon dioxide enthalpy is 71.47 kcal/kg (299.23 kj/kg).
This figure shows the different heat capacity of both gases:
 2. Remy’s Consumption 2. Remy’s Consumption
2.1 Monthly volume:
About 20 Tons
3. Heat Capacity Comparison
20Tons LCO2 equal to 16.76Tons LN2 per month.
4. LN2 advantage
4.1 Low pressure tank to store
4.2 Low pressure piping system
4.3 No block by potential “Dry Ice”.
4.4 More heat capacity, less volume.
|



